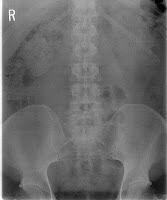
If you keep up with the blog, you know about the complications associated with metal on metal hip implants like Johnson & Johnson’s DePuy ASR. Yesterday, the New York Times Business Section had a report on the soaring number of complaints these hip replacement systems are receiving at the FDA.
Since January of 2011, the FDA has fielded more complaints about the metal on metal hip replacement systems than the previous four years combined. The DePuy ASR accounted for 75% of the complaints according to the New York Times complaint reviews.
Metal on Metal Hip Replacement News from the Hardison & Cochran Blog:
New York Times: Recalled Devices Mostly Untested, New Study Says- Hardison & Cochran Files First of Many DePuy ASR™ Cases
- DePuy Hip Replacement: Women More Likely To Need Revision Surgery Than Men According to Joint Registry
- The Implant Loophole: New York Times (DePuy Hip Replacement)
- MDL No. 2197 DePuy Orthopaedics Inc. ASR Hip Implant Products Liability Litigation
- Chicago NBC Affiliate News Story On DePuy Hip Replacement Recall
- ABC News Video: DePuy Hip Replacement
(HT: @StaceyEBurke)
* Photo courtesy of Simon Davison by way of Flickr Creative Commons




Leave a comment